The central vision of the initiative is the development and deployment of IT solutions to strengthen research and enhance patient care in university hospitals.
The goal is to ensure that locally developed IT solutions are interoperable with other systems. Furthermore, the new infrastructures must meet very high standards of quality, data protection and security. It is especially important to understand the aims, requirements, and concerns of diverse stakeholders – including scientists, doctors, patients, representatives of regulatory bodies, and many others – and to incorporate these insights into the initiative.
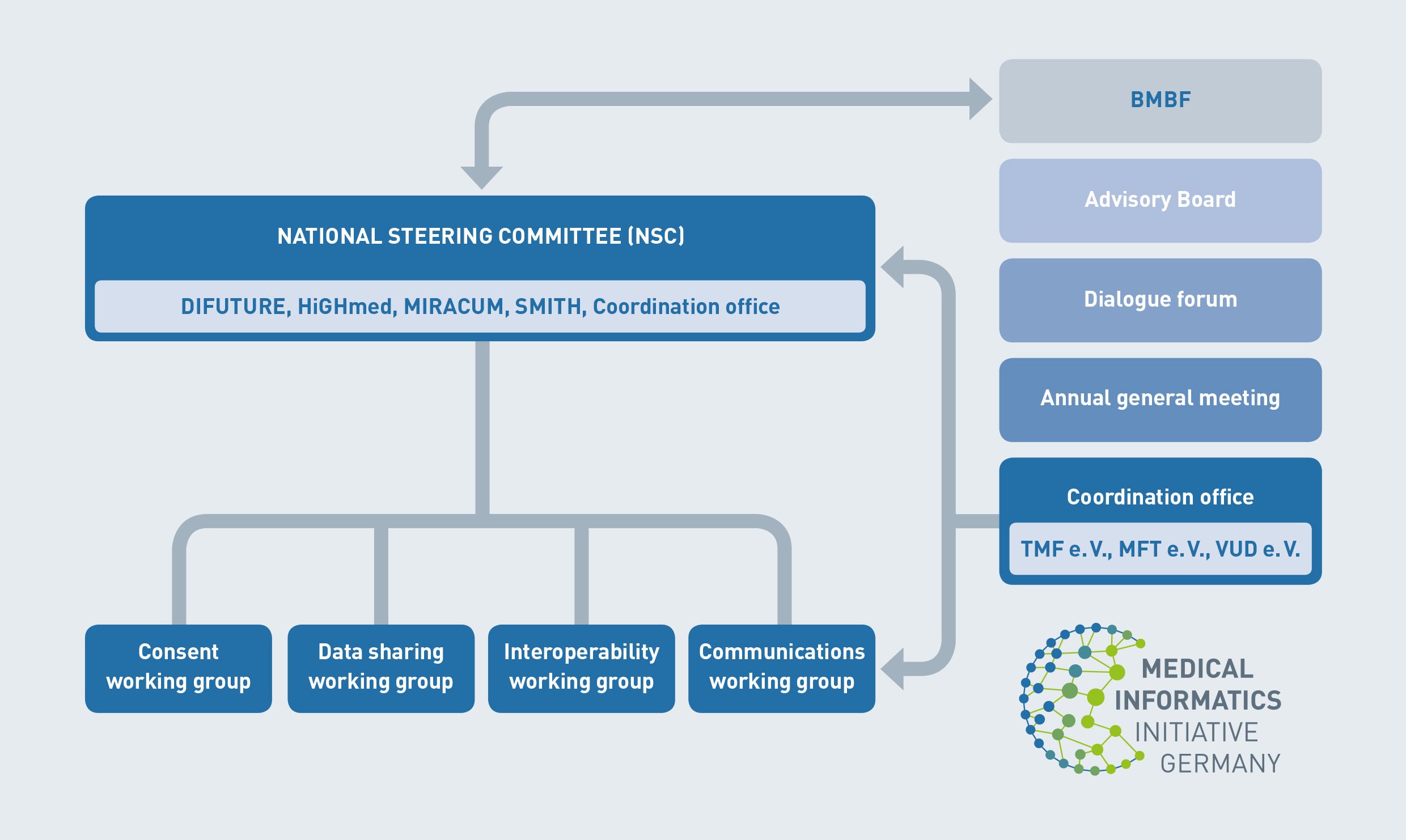
The core tasks of the medical informatics initiative will be carried out primarily by the university hospitals. Each consortium comprises a minimum of two university hospitals, plus various partners. In addition, the funding scheme allows additional organisations to join the consortia at a later stage.
All consortia are represented in the corresponding National Steering Committee, with the aim of aligning activities and agreeing parameters and to avoid siloed solutions and make significant headway at a national level.
These shared parameters are developed and discussed in dedicated working groups: consent, data sharing, interoperability and communication working group.
Additionally, the Dialogue Forum allows representatives from the various groups to exchange ideas and information. Participants include patient advocacy groups, policy makers, government agencies, research centres and other scientific institutions, associations, and healthcare IT industry organisations. The forum ensures that strategic planning is discussed, agreed and supported by all stakeholders.
The members of the Scientific Advisory Board (SAB) advise the National Steering Committee on overarching topics that either have an impact on the Medical Informatics Initiative as a whole or the external framework of reference.
Collaboration between and beyond consortia is supported by a coordination office jointly managed by TMF – Technology, Methods and Infrastructure for Networked Medical Research, MFT (Medizinischer Fakultätentag – German Association of Medical Faculties), and VUD (Verband der Universtitätsklinika Deutschlands – German Association of Academic Medical Centers).